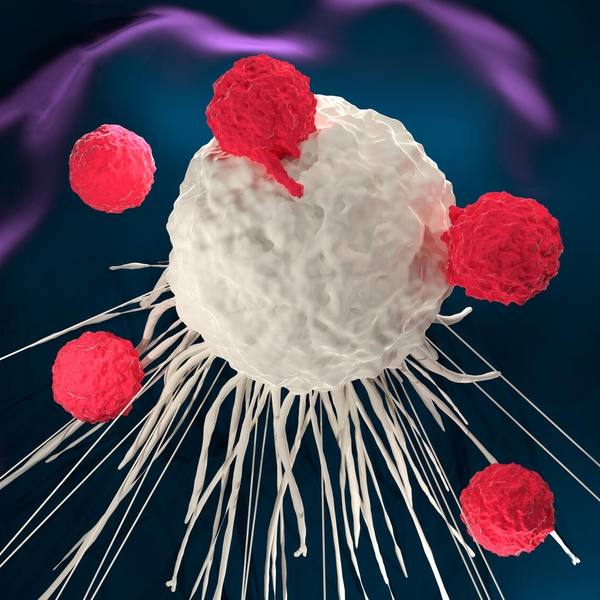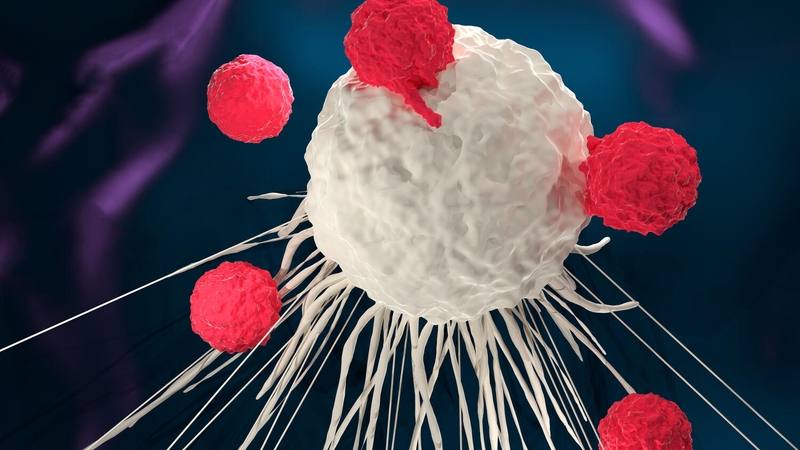
ROCHESTER, Minn. — Survival outcomes using chimeric antigen receptor-T cell therapy (CAR-T cell therapy) continue to be impressive for patients with some blood cancers.
“Five years ago, the survival rate for people with blood cancers was 10% to 15%. Nowadays, we are seeing a survival rate of 40%. The job is not done yet,” says Mohamed Kharfan Dabaja, M.D., a hematologist and director of blood marrow and transplantation and cellular therapies at Mayo Clinic Comprehensive Cancer Center. “We need to continue to bring new therapies, and to understand not only why it is not working on a certain patient but why it is in others and that will bring new knowledge about how to improve treating blood cancers.”
What is CAR-T cell therapy?
Immunotherapy uses the body’s immune system to fight cancer. CAR-T cell therapy is a form of immunotherapy in which healthcare professionals remove a person’s T cells — white blood cells known as lymphocytes that are involved in the immune system response — and genetically modify them to produce chimeric antigen receptors (CARs). These CAR-T cells are then infused back into the patient’s bloodstream, where they target and destroy cancer cells. CAR-T cell therapy is used primarily for blood cancers like multiple myeloma, leukemias and lymphomas.
Manufacturing CAR-T cells
Because of the encouraging results from CAR-T cells, Mayo Clinic researchers are persistent in searching for ways to expand their use. Saad Kenderian, M.B. Ch.B., a hematologist and oncologist at Mayo Clinic Comprehensive Cancer Center, and a team of Mayo Clinic researchers are investigating ways to make CAR-T cell therapy more accessible to patients through on-site biomanufacturing at Mayo Clinic’s campuses in Rochester, Minnesota, and Jacksonville, Florida.
“Being able to manufacture CAR-T cells in our lab will open the door for patients getting treatment faster, tailoring the treatment to their need, and potentially provide another option to patients who didn’t respond to the conventional, commercial-approved CAR-T cell therapy,” states Dr. Kenderian.
“Another strategy currently being tested in clinical trials uses allogeneic CAR-Ts, essentially CAR-T made from another source. This can provide a faster turnaround time for manufacturing, expand patient access and allow patients to receive CAR-T cell therapy at the time of need,” says Yi Lin, M.D., hematologist and oncologist at Mayo Clinic Comprehensive Cancer Center.
Allogeneic “off-the-shelf” CAR-T therapies are generated by healthy donors or gene-edited sources that have been genetically altered to reduce the possibility of rejection by the patient’s immune system. This approach is not yet approved by the Food and Drug Administration (FDA).
Using CAR-T cell therapy earlier during treatment for some blood cancers
While CAR-T cell therapy is initially offered as a therapy later during treatment for various blood cancers, recent research has shown it may be useful in some blood cancer cases earlier in a patient’s treatment plan. “CAR-T cell therapy in earlier lines of treatment has reported superior outcomes as compared to using it in later lines of therapy,” says Rafael Fonseca, M.D., hematologist at Mayo Clinic Comprehensive Cancer Center.
Next-generation CAR-T cell therapy trials are expanding into new tumor types like solid cancers and exploring new techniques such as using donor T-cells or immune cells other than T-cells, such as natural killer cells (NK cells), which are white blood cells that can destroy tumor cells.
Limiting the incidence and severity of side effects of CAR-T cell therapy
As with most cancer treatments, the side effects of CAR-T cell therapy can be unpleasant. They may include:
Cytokine release syndrome (CRS): This occurs when modified T-cells are introduced into a patient’s bloodstream. As the T-cells start targeting cancer cells, they release a large number of cytokines — proteins that can cause the immune system to overreact. This can include a fever, low blood pressure, muscle pains and flu-like symptoms.
Neurotoxicity: This is a potentially dangerous condition in which the immune response to the treatment affects the central nervous system. While the exact cause of neurotoxicity is not well understood, some studies suggest that it is partly related to the severity of CRS. Symptoms can include confusion, seizures and difficulty speaking or walking.
Blood disorders: CAR-T cell therapy can result in blood changes that can lead to anemia, thrombocytopenia (low platelet count) and other blood disorders. These effects are generally short-lived and resolve themselves over time, but they can be more severe in some patients.
Scientists are researching how using corticosteroids, and timing for when they are administered during treatment, could help lessen the side effects or make them more tolerable. “The future looks bright, and we will eventually get to that concept of not just treating cancer but treating cancer in a patient with the least amount of side effects and with maximum efficacy — that’s really where the gold is going to be,” says Dr. Kharfan Dabaja.
###
About Mayo Clinic Comprehensive Cancer Center
Designated as a comprehensive cancer center by the National Cancer Institute, Mayo Clinic Comprehensive Cancer Center is defining new boundaries in possibility, focusing on patient-centered care, developing novel treatments, training future generations of cancer experts and bringing cancer research to communities. At Mayo Clinic Comprehensive Cancer Center, a culture of innovation and collaboration is driving research breakthroughs that are changing approaches to cancer prevention, screening and treatment and improving the lives of cancer survivors.
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
Media contact:
- Kelley Luckstein, Mayo Clinic Communications, newsbureau@mayo.edu